Developing scalable Cell replacement therapies to treat incurable diseases for the world.
Our Market
We intend to be the benchmark for quality and affordability in cells therapies for our targeted conditions and expect to capture 1/6th of each of the following global markets:
| Dry AMD~ | USD 72 bn. |
| Retinitis Pigmentosa~ | USD 10 bn. |
| Idiopathic Pulmonary Fibrosis~ | USD 20 bn. |
| Product | Indication | In vitro studies | Proof-of-concept animal studies | GLP tox, safety and efficacy | First in Human trials |
|---|---|---|---|---|---|
| Eyecyte-RPETM | Geographic Atrophy | ||||
| Eyecyte-PRPTM | Retinitis Pigmentosa | ||||
| Aircyte-AEC | Idiopathic Pulmonary Fibrosis | ||||
| Neural Progenitor Cells | Parkinson’s Disease | ||||
| AAV- mediated gene augmentation platform | Inherited Retinal Dystrophies |

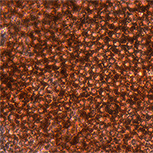
RPE cells – Phase
RPE cells – Immunocytochemistry
(RPE65+MITF+DAPI)
Our Lead Asset
Eyecyte-RPETM replaces lost retinal pigment epithelium cells. It is designed to restore sight for patients in the early stages of Age-Related Macular Degeneration and arrest losses for those in the later stages.
The product is allogenic and administered by sub-retinal injection.
Application for first in human trials with the Indian regulatory authorities is scheduled for Q2, 2023.
We are one of six players globally developing this solution.
“Eyecyte-RPETM provided significant beneficial / efficacious effects on the degenerating retina in RCS rats without any significant safety concerns, suggesting this cells type may have substantial therapeutic value in human retinal degenerative disease. In comparison with other published cells transplantation studies from companies that have moved forward to achieve IND approval for early phase clinical trials, Eyecyte-RPETM appears to have similar efficacy to that of XXXXX a leading cells therapy company in the US and slightly better than XXXX a top ten pharma company headquartered in Japan. Eyecyte-RPETM appears on par or better than each of the previous companies that have achieved IND approval. What has been achieved in terms of efficacy with Eyecyte-RPETM in this study should be regarded as very promising and is likely to match or exceed competitor’s current cells products.”

Casey Eye Institute
at Oregon Health and Science University
Our Other Assets
Eyecyte-PRPTM replaces lost photoreceptor cells. It is proven in treating Retinitis Pigmentosa in animals. Extensive animal testing is scheduled to begin in the second half of 2023 with an aim to move this product into clinical stage in second half of 2024.
We are one of five players globally developing this solution.
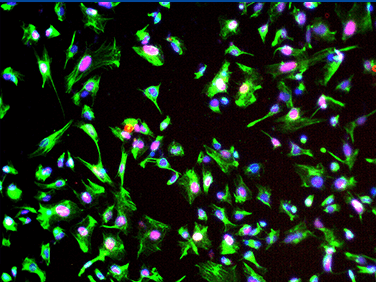
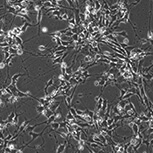
PRP cells – Phase
PRP cells – Immunocytochemistry
(NRL2+RECOVERIN+DAPI)
Aircyte-AEC restore lung function for patients suffering from Idiopathic Pulmonary Fibrosis. The product is delivered to the alveolar surfaces by an intra-bronchial inhalation. Proof of concept demonstration of animal efficacy has been done and the product will soon enter extensive animal testing.
We are the leading global player developing this solution.

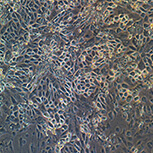
Lung epithelial cells – Phase
Lung epithelial cells – Immunocytochemistry
(ARL13B+ZO1+DAPI)
